Abstract
Fanconi anemia is the most common inherited bone marrow failure syndrome characterized by impaired DNA repair pathway. Patients with Fanconi anemia often present with congenital anomalies and up to 70% of patients develop bone marrow failure (BMF) during the first decade of life, with high risk of progression to myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). While high penetrance of BMF phenotype is a key characteristic of Fanconi anemia, patients may have variable onset and severity. Despite extensive research, there are no available data whether affected siblings with Fanconi anemia have a similar or different clinical course.
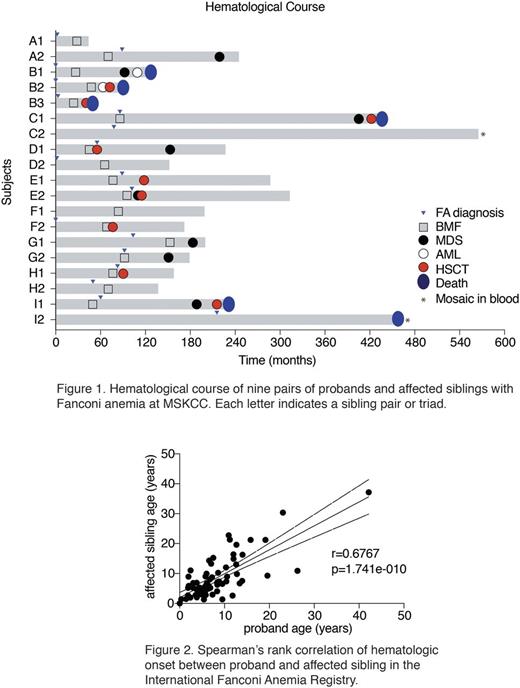
First, we reviewed medical records of 9 sets of probands and affected siblings with Fanconi anemia who were seen at the Memorial Sloan Kettering Cancer Center (MSKCC) in the last 30 years (Figure 1). As longitudinal and detailed clinical information was available for these sibling pairs, we collected data by retrospective chart review on the onset of BMF, MDS or AML, time to hematopoietic stem cell transplantation (HSCT), the presence of any congenital anomaly, microcephaly, renal anomaly, low birth weight, short stature, learning disability and frequent infections between older and younger siblings. After observing a signal of positive correlation in BMF onset between siblings, we moved onto reviewing additional sibling pairs in the International Fanconi Anemia Registry (IFAR), where additional 62 sibling pairs with available hematologic onset information were registered. IFAR defines hematologic onset as the onset of BMF, MDS or AML.
The Spearman rank correlation coefficient was computed to assess correlation between sibling pair's clinical manifestation for those seen at MSKCC. We observed a positive correlation in the time to bone marrow failure, defined by development of any peripheral blood cytopenias, between sibling pairs, although the p value was borderline due to small sample size (correlation coefficient r=0.75, p=0.05, number of pairs = 7). We excluded two pairs of siblings in which one of the siblings was known to have blood mosaicism. The sample size was too small to compute statistics for the time to MDS/AML or time to HSCT. We also calculated the Cohen's kappa coefficient to determine whether a particular clinical event was likely to occur in both siblings or neither of siblings. We found that kidney anomaly (kappa coefficient = 0.5714; 95% CI 0.0982 - 1) and microcephaly (kappa coefficient = 1; 95% CI 1-1) showed good agreement between siblings, whereas we did not find good agreement between siblings in skeletal anomaly, cardiac anomaly, low birth weight, short stature, growth retardation, learning disability or frequent infections. To assess correlation of hematologic onset in all available sets of probands and affected siblings, the Spearman's rank correlation was calculated on total 69 sibling pairs. We found a positive correlation in the hematologic onset, defined as time to bone marrow failure, MDS or AML, whichever comes first (correlation coefficient r=0.6767, p <0.0001; Figure 2).
In summary, affected siblings with Fanconi anemia tend to develop hematologic manifestations at similar ages with a median age difference of 2.18 years (range 0 - 15.36 years; 95% CI 2.61 - 4.17 years). Among other clinical manifestations, kidney anomaly and microcephaly appeared to occur concordantly between siblings. We plan to reach out to other institutions to enhance collaboration and increase number of research participants.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal